Certifications Our sera

Our serum, traceable to the source
When scientists select their sera, the geographical source and the products traceability are of paramount importance.
Traceable to the source
When scientists select their sera, the geographical source and the products traceability are of paramount importance.
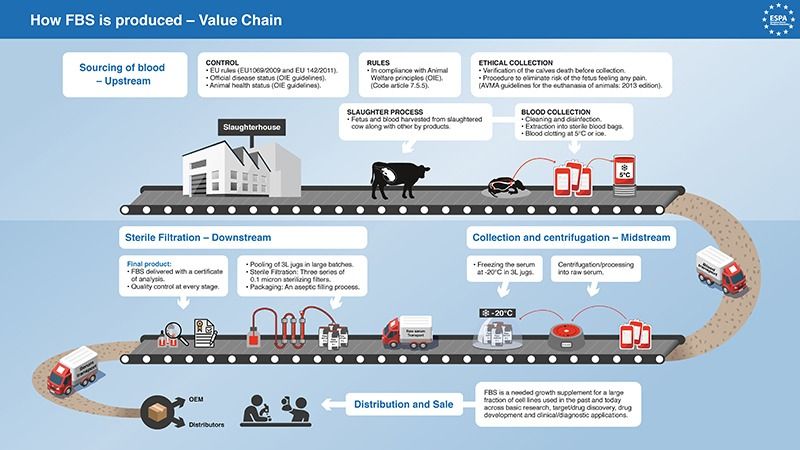
Our Fetal Bovine Serum is extracted from clotted whole blood collected aseptically from the fetus via cardiac puncture.
Biowest rigorously controls each manufactured batch:
- From the serum collection,
- During its treatment and production,
- To the final packaging on our premises.
Biowest analyses, classifies, and tests its products before shipment to customers worldwide.
Our Quality System can trace raw materials back to the original supplier and slaughterhouses where they were collected.
Biowest’s system of vertical integration provides origin certification and full sera traceability.

FBS Sources Worldwide
Biowest supplies a wide range of sera origins including sources from South America as well as from the European Union (EU) and from sources approved by United States Department of Agriculture (USDA).
The choice of FBS origin is determined by customer needs, import requirements and worldwide supply.
Professionals will find Biowest to be the ideal partner for meeting their requirements, such as choosing the origin of serum which provides optimal performance and results.
FBS (Foetal Bovine Serum) is considered to be an animal by-product which is not intended for human consumption.
Biological safety is controlled by EU rules on animal by-products:
- Regulation EU 1069/2009 and Regulation EU 142/2011
- Official disease status BSE, FMD, etc.
- Individual countries are monitored for their animal health status by the World Organisation for Animal Health (WOAH)
Filtration and packaging
Raw pooled Fetal Bovine Serum is filtered by three series of 0.1 micron sterilizing filters. The sterile filtered serum is true-pooled to ensure homogeneity.
Biowest products are packaged via an aseptic filling process.
We ensure that the industrial sterility standards at a level of 10-4 are met at every step of manufacturing.
Our products demonstrate a bacterial and fungal contamination level of less than 1 of 10,000 units throughout fabrication.
The highest level of sterility assurance (≥10-6) cannot be achieved without terminal sterilization.
The filtration and dispensing are performed under positive pressure in HEPA-filtered environmentally controlled rooms.
Each Batch is delivered with a Certificate of Analysis.
Biowest Quality Certification
The sterility test procedure is based on either European or US Pharmacopoeia, depending on the location of the final filtration.
To achieve the highest Sterility Assurance Level, a representative number of samples from each production batch is selected for sterility testing before distribution.
Biowest sera are all tested for the absence of aerobic and anaerobic bacteria, fungi, and yeast.
Each final product batch of sera are tested for Mycoplasma.
The sera are tested for the absence of Mycoplasma using a cell culture assay in Axcell Biotechnologies media by culture method. Our test is accurate within the limits of the detection method used.
Depending on the species of the serum, each batch of serum is tested for adventitious viruses using cell culture techniques.
Sera are tested for the absence of the indicated viruses by inoculation with GBK cells. The detection of virus is made by indirect immunofluorescence. Antibody Testing : the presence of specific antibodies is detected utilizing an ELISA Assay. For exemple, the serum from equidae is tested for the presence of Equine Infectious Anemia antibodies.
All Biowest pH meters are calibrated daily with standard solutions.
Osmolality is determined by a lowered freezing temperature, following European Pharmacopeia EU Ph. 2.2.35
The osmometer is calibrated using traceable standards.
All Biowest sera are tested to determine and quantify endotoxin levels employing a chromokinetic – quantitative test - method D of the European Pharmacopoeia EU Ph. 2.6.14.
The residual haemoglobin concentration in each batch is determined by a quantitative and colorimetric assay to verify that the proper collection and processing procedures have been followed.
Protein detection | Methodology |
Protein | Biuret Colorimetry |
Albumin | Immunoturbidimetry |
Globulin | Immunoturbidimetry |
Test | Method |
ALAT (SGPT) | UV Kinetic at 37°C |
Alkaline Phosphatase | Colorimetric Kinetic at 37°C |
ASAT (SGOT) | UV Kinetic at 37°C |
Bilirubin | DPD / Cafeine Colorimetry |
Calcium | Arsénazo Colorimetry |
Gamma GT | Colorimetric Kinetic at 37°C |
Cholesterol | Cholesterase Trinder Colorimetry |
Creatinine | Colorimetric Kinetic (Jaffé) |
Chloride | Indirect Potentiometry |
Glucose | Hexokinase UV |
Iron | TPTZ Colorimetry |
Lactate Dehydrogenase (LDH) | UV Kinetic at 37°C |
Phosphorus | Phosphomolybdate Colorimetry |
Potassium | Indirect Potentiometry |
Sodium | Indirect Potentiometry |
Tryglicerides | Glycerokinase Trinder Colorimetry |
Urea | Urease UV |
Uric Acid | Uricase Trinder Colorimetry |
Each batch of serum is tested for IgG using an ELISA method.
Each batch of serum is manufactured for maximum in vitro growth support of particular cell lines.
As well as conforming to our strict Quality Control specifications, each batch of sera fulfils threeimportant performance criteria:
- Growth Promotion
- Plating Efficiency
- Cloning Efficiency
The biological performance is assessed using cell culture medium supplemented with a final concentration of 10% serum. During the test period, cultures are examined under microscope for morphological abnormalities that could indicate toxic components in the serum.
The following cell lines are used to measure growth potential and functionality:
Cell Line | Type | Species |
HELA | Cancer | Human |
L929 | Fibroblast, Macrophage | Mouse |
SP2/0-AG14 | Lymphoma | Mouse |
MRC-5 | Lung | Human |
Bovine Spongiform Encephalopathy (BSE) is tested for bovine derived material. According to the European Regulation EC n° 999/2001, animals are tested for BSE before the corresponding blood is allowed to be processed. The EU is a pioneer in BSE testing and individual identification of animals through ear tagging. This ensures the best possible traceability and the lowest BSE risk. Consequently the EU origin is the first choice of researchers in Japan and other selective markets.
Japan and other select markets make the EU sera origin their first choice.
Test | Method |
Prionics | Western Blot |
Bio-Rad | ELISA |
Note:
Even though the World Animal Health Organization confirms that blood and blood products like FBS do not pose a risk for the transmission of BSE, some countries still have BSE requirements for FBS
- See WOAH – Terrestrial Animal Health Code (2022)
All Biowest products have labels indicating suitable storage conditions, batch number, and the expiry date.
Biowest guarantees optimal serum performance when the product is properly stored:
- Animal sera and plasma require storage at -20°C.
- Respect the expiry date.
- The shelf life for animal serum is 60 months, and for animal plasma is 48 months.
Intended Use

These products are intended for research applications or further manufacturing only.
It is the end user’s responsibility to qualify these products for their specific application.
These products are not for use in therapy, human or veterinary applications nor as an Active Pharmaceutical Ingredient or for food or animal feed.
Their safety and efficiency have not been established for diagnostic or other clinical uses.
