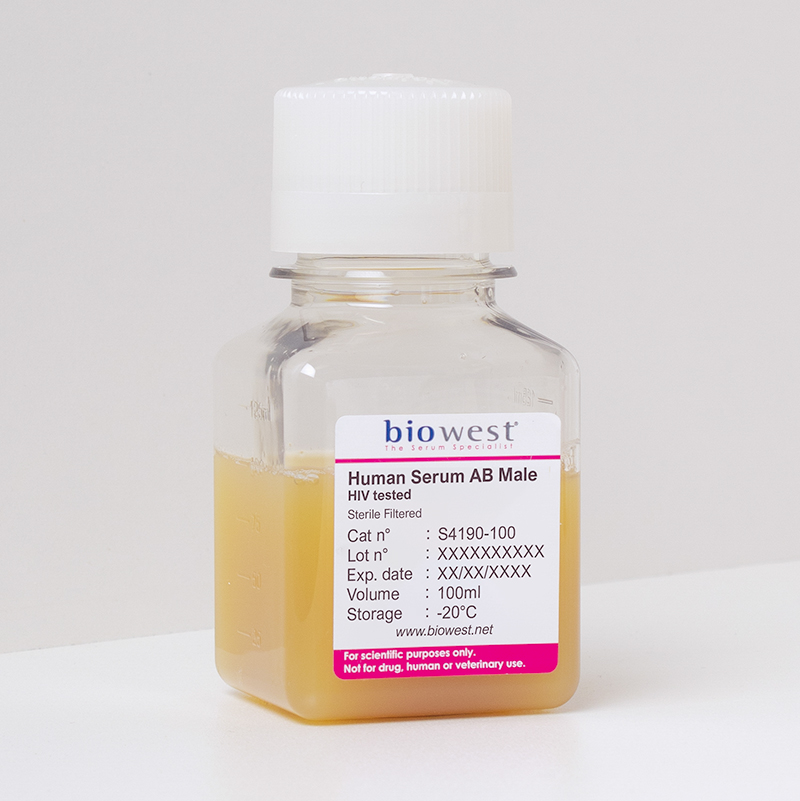
Human Serum AB male HIV tested – S4190
Human Serum AB male HIV tested – S4190
Human Serum is the liquid part of the human blood depleted of the cellular fraction and fibrinogen/coagulation factors. It is obtained after natural coagulation process of human blood, without anticoagulant, from volunteer donors.
Serum from AB blood type are free of antibodies against type A and B antigens, minimizing immunoreactivity. This serum is used in fields of study where immunoreactivity is important to control such as cell therapy or transplantation
The serum is sourced from pool of AB male blood
Sterility, endotoxin and hemoglobin levels, osmolality and cell culture performance are tested for each batch. HIV Type 1 and 2 (HIV1/2), Hepatitis B (HBS), Hepatitis C (HCV) are screened for each human serum batch.
Origin: Europe, USA (approuved countries)
The serum is collected or imported and treated in agreement with the European regulations
Biowest guarantees the accuracy of geographic origins
All sera can be treated, please inquire about the possibilities
Other packagings available on request
Important: Products of human origin should be considered as potentially infectious and handled accordingly.
End users of the products must comply with the legislation in force in their country for any use and/or sale of products of human origin.
Cell culture
Western Blot, ELISA
The product is intended for in vitro, laboratory use only. Do not use in therapy, for human or veterinary applications.
The handling and sale of human-derived products in Europe are requires authorization.
In France, this authorization is granted by the Ministry of Higher Education, Research, and Innovation after the submission of a detailed file. Biowest holds the following authorizations :
- Import and export activities for scientific purposes of organs, tissues, and cells derived from the human body and their derivatives, blood, its components, and derived products.
- Conservation and preparation activities of human body elements for their transfer for scientific use.
In order to be able to sell these products to its customers, Biowest must register its clients in its import/export file and submit this updated document to the authorities for validation. The validation process takes between 1 and 3 months.
The following information is essential for this registration : name, delivery address, phone number, and intended use of the products.
Biowest cannot be held responsible for misuse of its human products by end users.
Please contact us for MSDS in other languages
In the service of science since 1987
Founded in 1987, Biowest is the European leader in the collection of animal sera, providing the widest range of sera and cell culture media available on the market.
Our products are employed in the production of biopharmaceuticals and vaccines and in the academic, governmental and industrial research sectors in fields spanning the areas of cell biology, genomics, proteomics, virology, immunology, innovative drug research and toxicology.
All our engagements